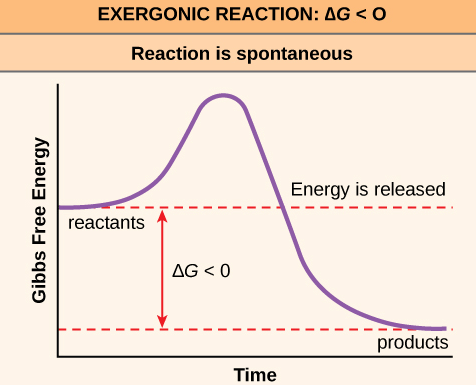
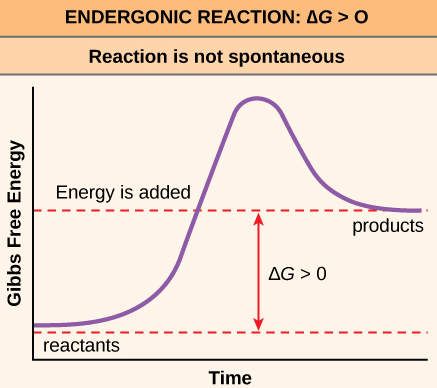
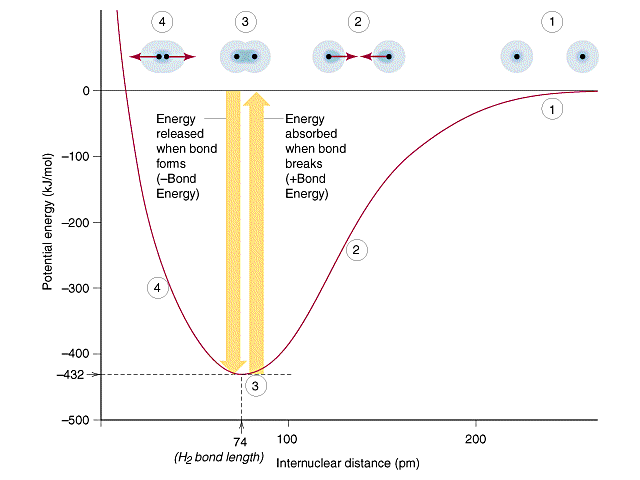
Question 11
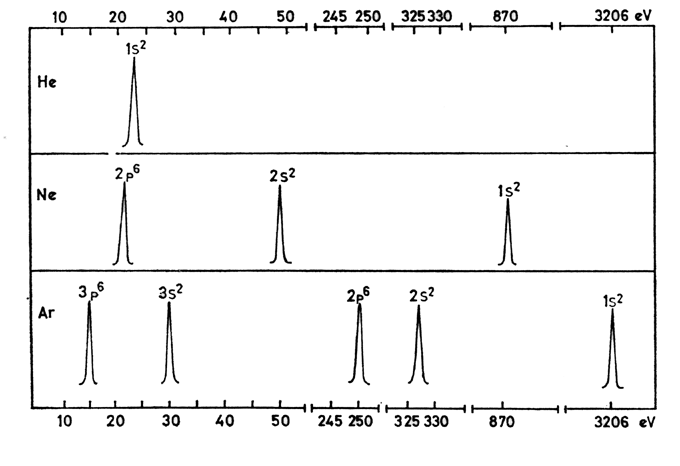
- The further away an electron is from the nucleus, the less binding energy that the incoming photons need to overcome, and as a result, the more kinetic energy the electron will have after it is ejected.
Question 20
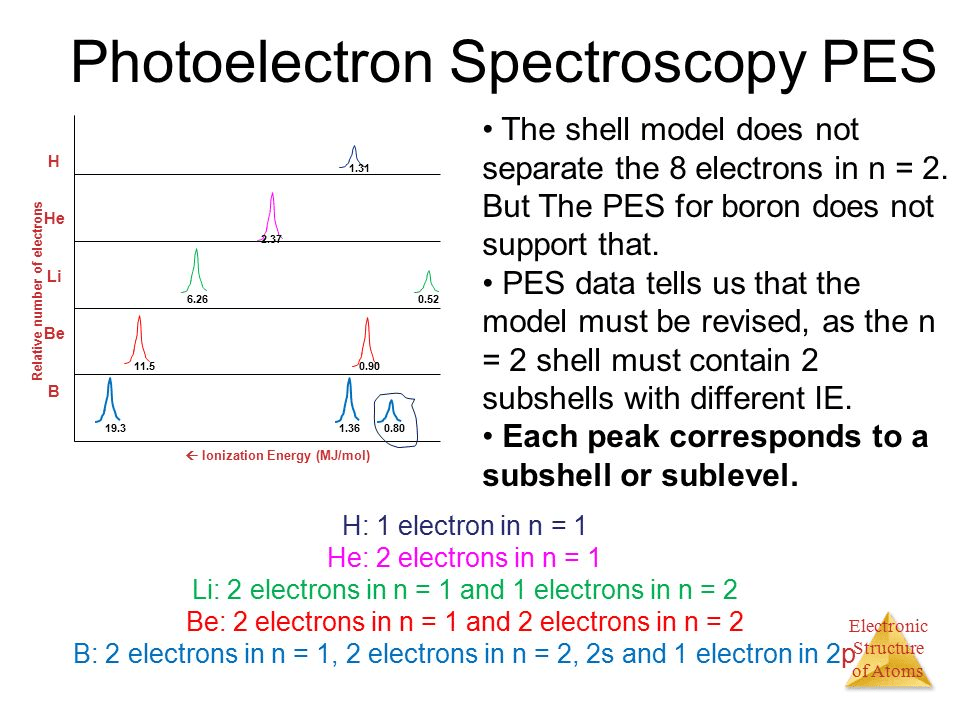
Question 21
The bond length always corresponds to the point where the potential bond energy (a balance of the attraction and repulsion forces between the two atoms) is at it’s minimum value.
Question 23
- Weak acids resist changes in pH more effectively than strong atoms because so many molecules of weak acid are undissociated in solution. The base must cause those molecules to dissociate before affecting the pH significantly.
Question 25
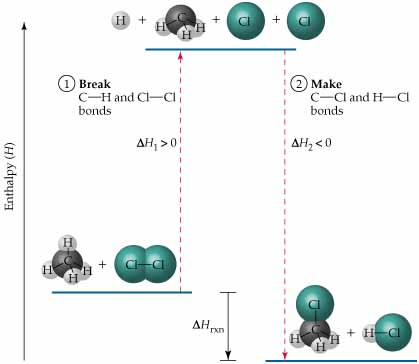
When calculating enthalpy, the total energy is always the bonds broken (reactants) minus the bonds formed (products). The more positive this value is, the more energy there is in the reactants compared to the products.
Question 36
- The outermost s-block electrons in a transition metal tend to be lost before the d-block electrons do.
Question 40
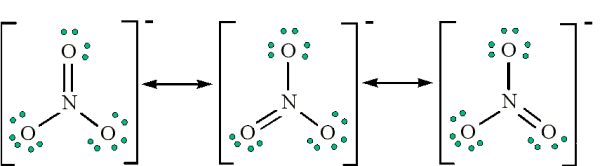
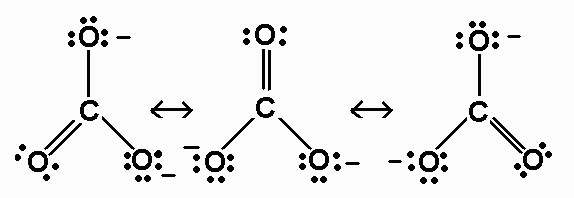
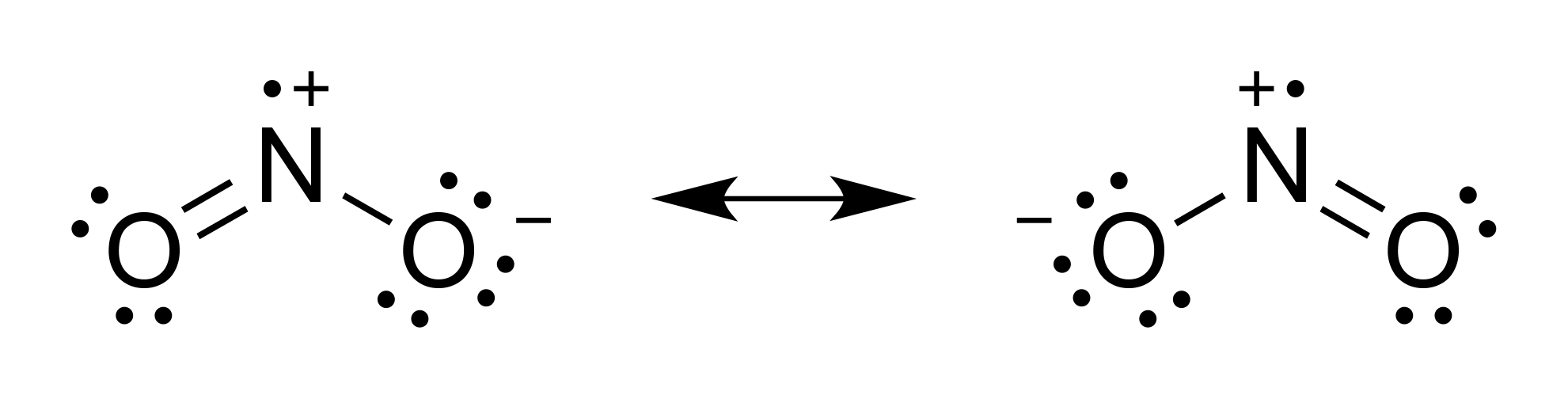
Question 44

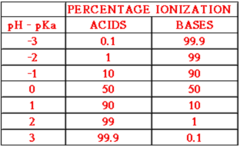
Question 49
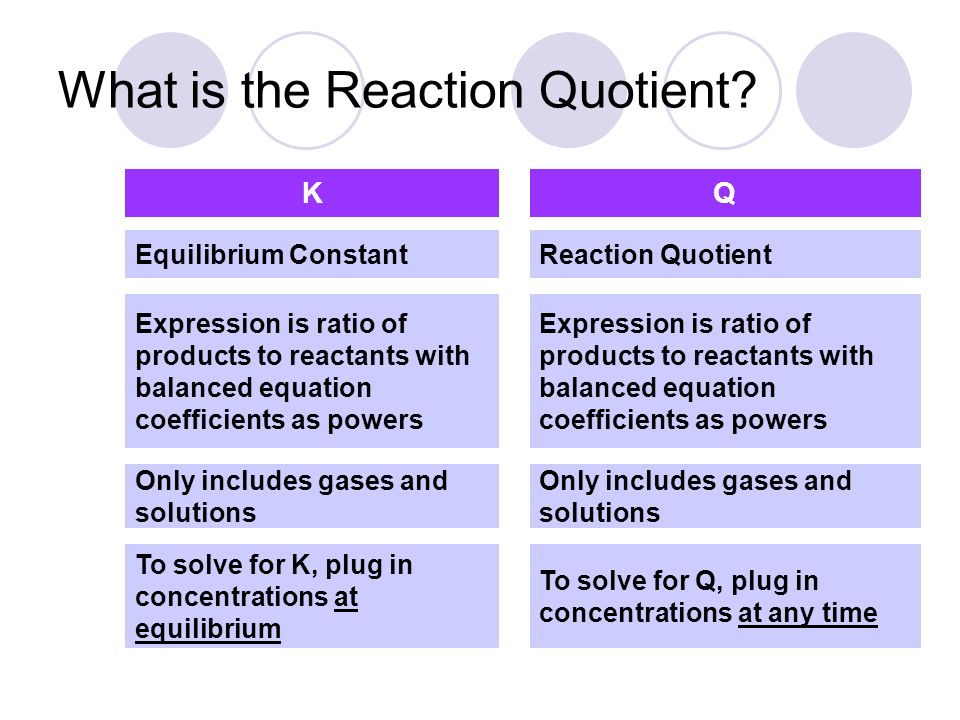

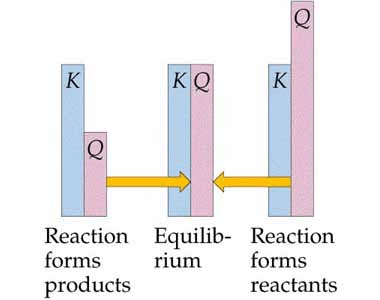
Question 53
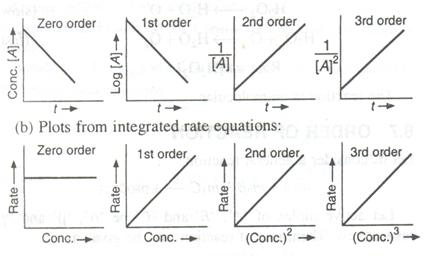
Question 55
- The strength of an atom’s magnetic moment increases with an increase in the number of unpaired electrons.
Question 60